Moderna Booster Vaccine Shot
Moderna said the booster even. A company study of 344 people gave them a 50-microgram shot six months after their second dose and levels of virus-fighting antibodies jumped.
With Pfizer Vaccine Boosters Now Available What S Next For Moderna And J J What To Know Cnet
The company said that would trigger fewer uncomfortable shot reactions such as fever and achiness while also leaving more vaccine.

Moderna booster vaccine shot. Modernas current vaccine shot is a 100-microgram dose compared with Pfizers 30-microgram dose. Ad FDA has authorized the Moderna COVID-19 Vaccine under an Emergency Use Authorization. Learn more about the facts behind the COVID-19 Vaccine.
The Moderna vaccine has been authorized by the FDA for use in people who are at least 18 years old. At this time people who got the Moderna vaccine are not eligible for a booster shot. If you received a Moderna COVID-19 vaccine.
2 shots 28 days apart Some immunocompromised people should get 3 shots. Currently 2 vaccines are authorized and 1 is approved in the US. A third dose of the Moderna vaccine given six months after the initial two doses significantly boosts immunity according to data the company submitted to the Food and Drug.
The CDC recommends a third dose of an mRNA COVID-19 vaccine including the Pfizer-BioNTech and Moderna COVID-19 vaccines for some people with weakened immune systems such as those who have had an organ transplant. 2 days agoAn expert committee on Thursday recommended a booster dose of Modernas anti-Covid vaccine in the United States for certain at-risk groups a month after making a similar decision for the Pfizer shot. More data on the effectiveness and safety of Moderna booster shots.
Moderna vaccine key takeaways. FDA panel recommends Moderna booster shots 0320. Moderna submitted data to the FDA seeking evaluation for its booster shot on Sept.
Type of Vaccine. 2 days agoVaccine experts advising the Food and Drug Administration voted 19-0 to recommend authorization of an extra dose of Modernas Covid-19 shot a key step in making booster doses. Because residents in long-term care settings external icon live closely together in.
Learn more about the facts behind the COVID-19 Vaccine. 1 day agoThe FDA may soon approve a Moderna booster after a unanimous vote by the Vaccines and Related Biological Products Advisory Committee. Ad FDA has authorized the Moderna COVID-19 Vaccine under an Emergency Use Authorization.
But Moderna says a single 50-microgram shot should be enough for a booster. Has however already approved both Pfizer and Moderna boosters for certain people with weakened immune systems such as cancer patients and transplant recipients. 2 days agoA key FDA advisory committee unanimously recommended Thursday giving booster shots of Modernas Covid-19 vaccine to people ages 65 and older and other vulnerable Americans.
Residents aged 18 years and older of long-term care settings should get a booster shot of Pfizer-BioNTech vaccine. An outside panel of the Food and Drug Administrations vaccine experts voted unanimously to endorse Modernas request to roll out booster shots. If you got the Johnson Johnson vaccine as your first COVID-19 shot a booster dose of either the Moderna or Pfizer-BioNTech vaccine apparently could.
As for the dose initial Moderna vaccination consists of two 100-microgram shots. This vaccine has a 941 efficacy rate can be. We are pleased to initiate the submission process for our booster candidate at the 50 µg dose with.
People with weakened immune systems might not develop enough immunity after vaccination with two doses of an mRNA COVID-19 vaccine. Cutting the Moderna doses in half could help reduce the risks of side effects from the booster. All approved or authorized COVID-19 vaccines are effective.
Ad Do not wait to be protected. 2 days agoModerna is seeking authorization of a booster that contains 50 micrograms of vaccine half of strength of its regular dose but still higher than the Pfizer BioNTech shot using similar technology. 1 day agoOn Thursday the Food and Drug Administration FDA moved one step closer to giving the green light to Modernas COVID-19 vaccine booster shots when an advisory committee.

Moderna Says Covid 19 Boosters Effective Against Variants

Moderna Booster Shot For Covid 19 Could Be Delayed Wbtw

Moderna Is Developing Vaccine Booster Shot To Fight South Africa Covid Strain Youtube

Moderna Asks Fda To Authorize A Booster Shot Of Its Covid 19 Vaccine Coronavirus Updates Npr

Moderna Plans To Have Covid Vaccine Booster Shot Ready By Fall Cbs News
What To Know About Covid Booster Shots If You Re Immunocompromised Shots Health News Npr
With Pfizer Vaccine Boosters Now Available What S Next For Moderna And J J What To Know Cnet
Covid 19 Booster Shots Will Roll Out In September In The U S Coronavirus Updates Npr

Future Of Public Health Role Of Covid 19 Vaccine Boosters News Uw Health
Moderna Covid Vaccine Booster Shot Update New Fda Panel Details To Know Cnet

Pfizer Booster Can Be Given After 6 Months European Drug Agency Says The New York Times

Us Sets The Stage For Covid Booster Shots For Millions Wkbn Com

Boosters For Moderna And J J Recipients Not Up For Debate At C D C Panel The New York Times
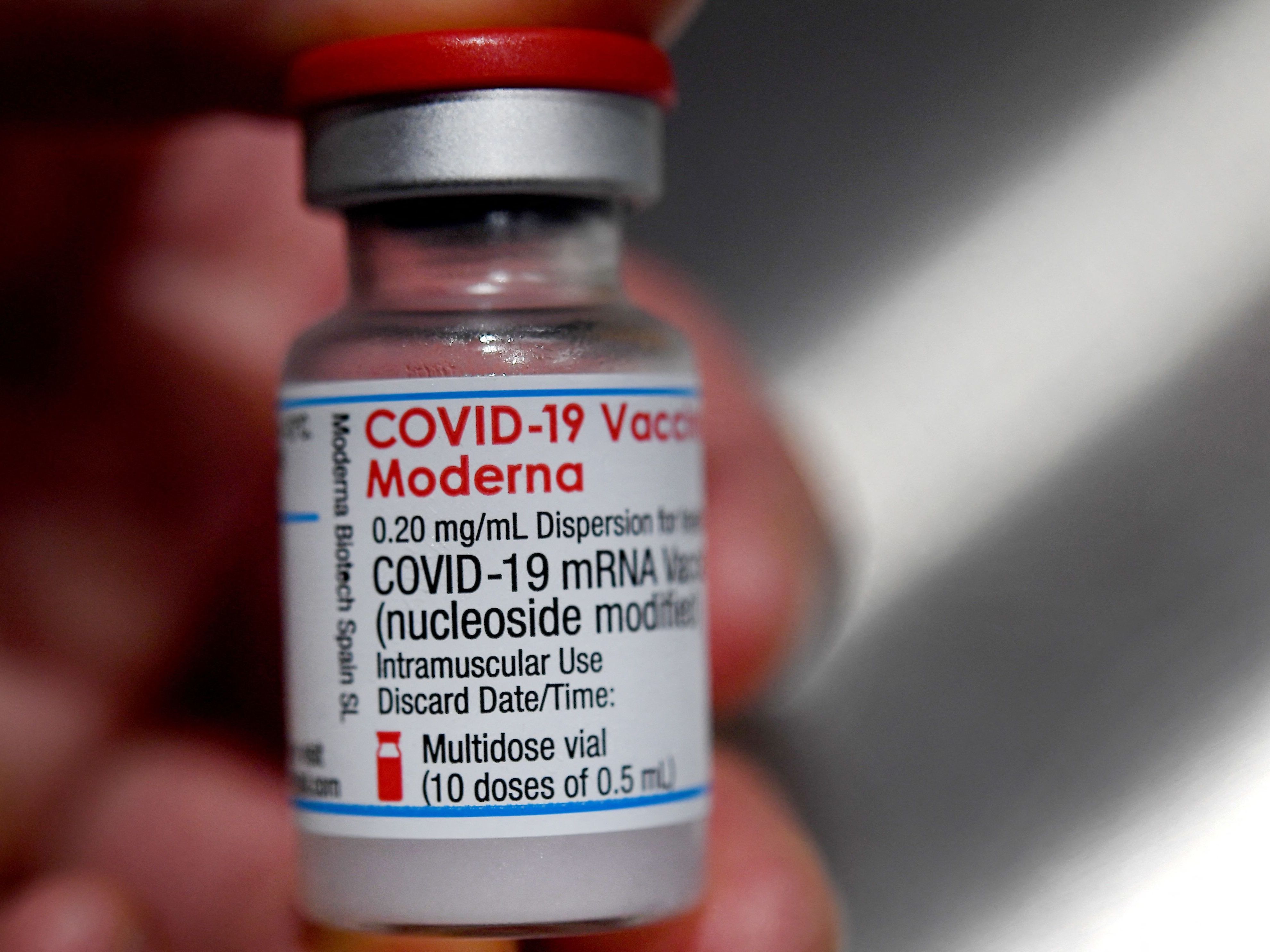
Moderna Says Its Vaccine Is Effective Against The Delta Variant Coronavirus Updates Npr
Fda Considering Authorization For Half Dose Moderna Covid 19 Booster Shot Al Arabiya English

Next On Fda S Agenda Booster Shots Of Moderna J J Vaccines 660 News

Covid Booster Shot Moderna Says Vaccine Generates Promising Immune Response Against Variants

When Should I Get A Covid Booster Shot Wjhl Tri Cities News Weather
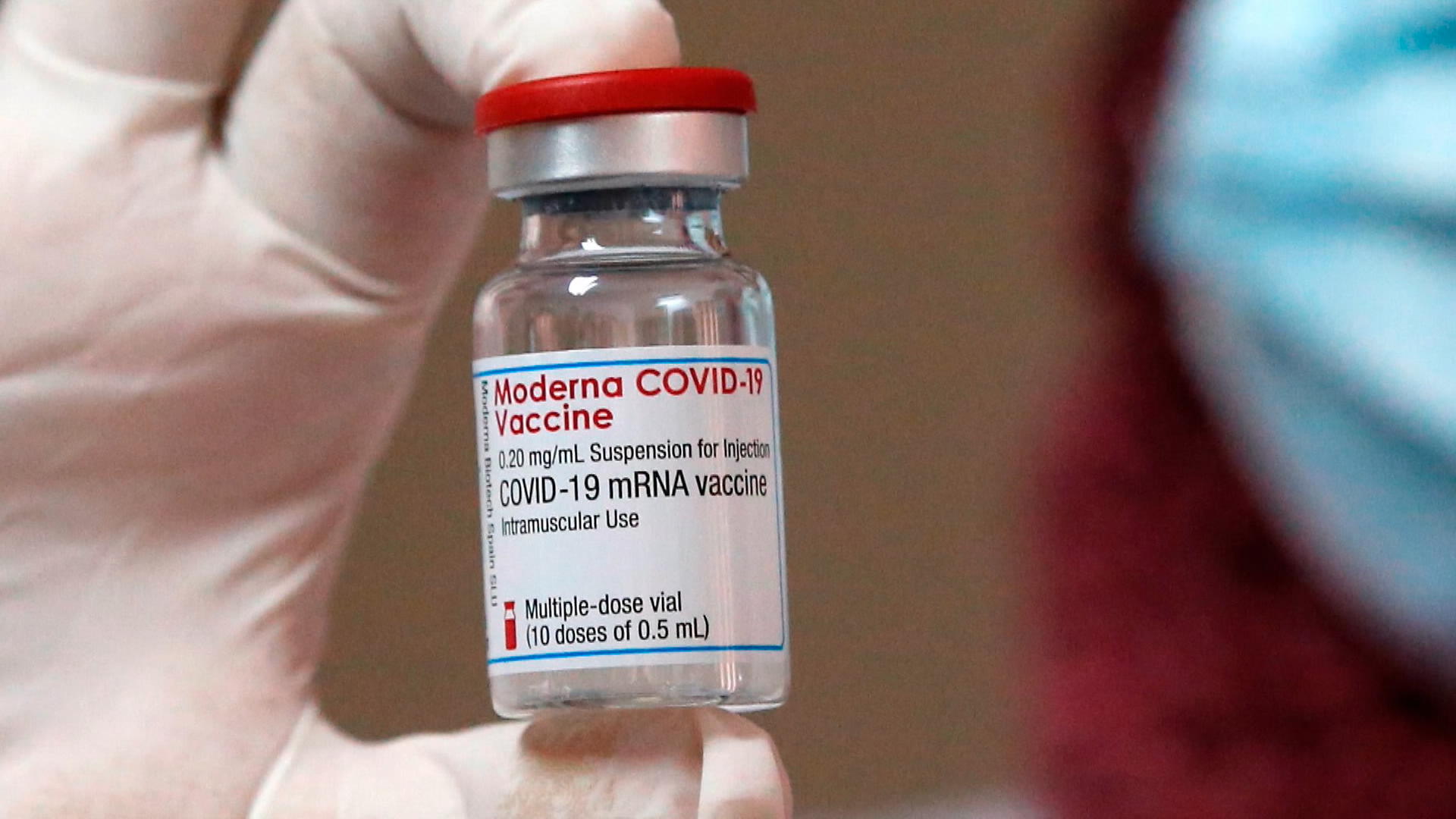
Moderna Covid Booster Shots Prove Effective Against Variants Bloomberg